Four people have reportedly had to have their eyeballs surgically removed.
The number of deaths in an outbreak is related to contaminated recalled eye drops increased and more people lost their sight.
According to a update released by the Centers for Disease Control and Prevention on Tuesday, the death toll rose from one to three.
Furthermore, at least eight people were blinded and four people were surgically removed.
The CDC did not provide any information in its update about the affected patients including names, ages, genders or where they live.
More than 10 different brands of artificial tears have been recalled. Most of the cases have been linked to EzriCare and Delsam Pharma eye drops, made by India-based Global Pharma Healthcare.
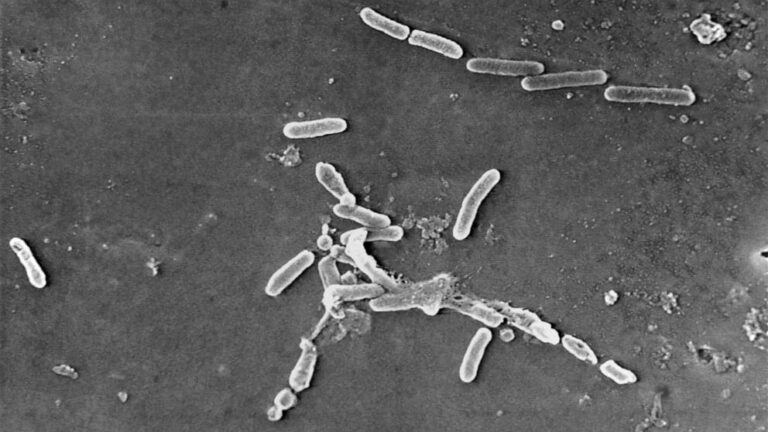
According to the CDC, the eye drops were contaminated with an antibiotic-resistant form of Pseudomonas aeruginosaan aggressive bacterium.
Pseudomonas is a type of bacteria found in the environment, with P. aeruginosa most commonly causing infections in humans.
Infection is common in health care settings and spreads from improper hygiene due to dirty hands or medical equipment and surfaces that have not been properly cleaned.
P. aeruginosa is resistant to many types of antibiotics and has caused about 32,600 infections in hospitalized patients in the US and an estimated 2,700 deaths, according to the CDC.
The strain linked to the outbreak, however, has not been reported in the United States before, the CDC stated in its update.
As of March 14, 68 people across 16 states have been infected with P. aeruginosa. Of those cases, 37 were linked to four health care clusters.
Last month, the US Food and Drug Administration gave a warning, supported by the CDC, urging health care personnel and the public not to purchase EzriCare Artificial Tears or Delsam Pharma’s Artificial Tears due to potential bacterial contamination.
After warning, Global Pharma Healthcare issued a voluntary recall of both products, notifying distributors and advising wholesalers, retailers and customers with discontinued products.
Not long after, the FDA also recommended that Global Pharma recall Delsam Pharma’s Artificial Eye Ointment, which the company agreed to. So far, no reports of infections have been linked to this product.
The CDC warns anyone with symptoms of an eye infection who has used EzriCare or Delsam Pharma eye drops to seek medical care immediately.
Such symptoms include yellow, green, or clear discharge from the eye; eye pain or discomfort; red eyes or eyelids; feeling something in the eye; increased sensitivity to light; and blurred vision.
The CDC did not immediately respond to ABC News’ request for comment.